Lamisil AT Spray Prescribing Information
Package insert / product label
Generic name: terbinafine hydrochloride
Dosage form: spray
Drug class: Topical antifungals
Medically reviewed by Drugs.com. Last updated on Mar 25, 2024.
Active ingredient
Terbinafine hydrochloride
Indications and Usage for Lamisil AT Spray
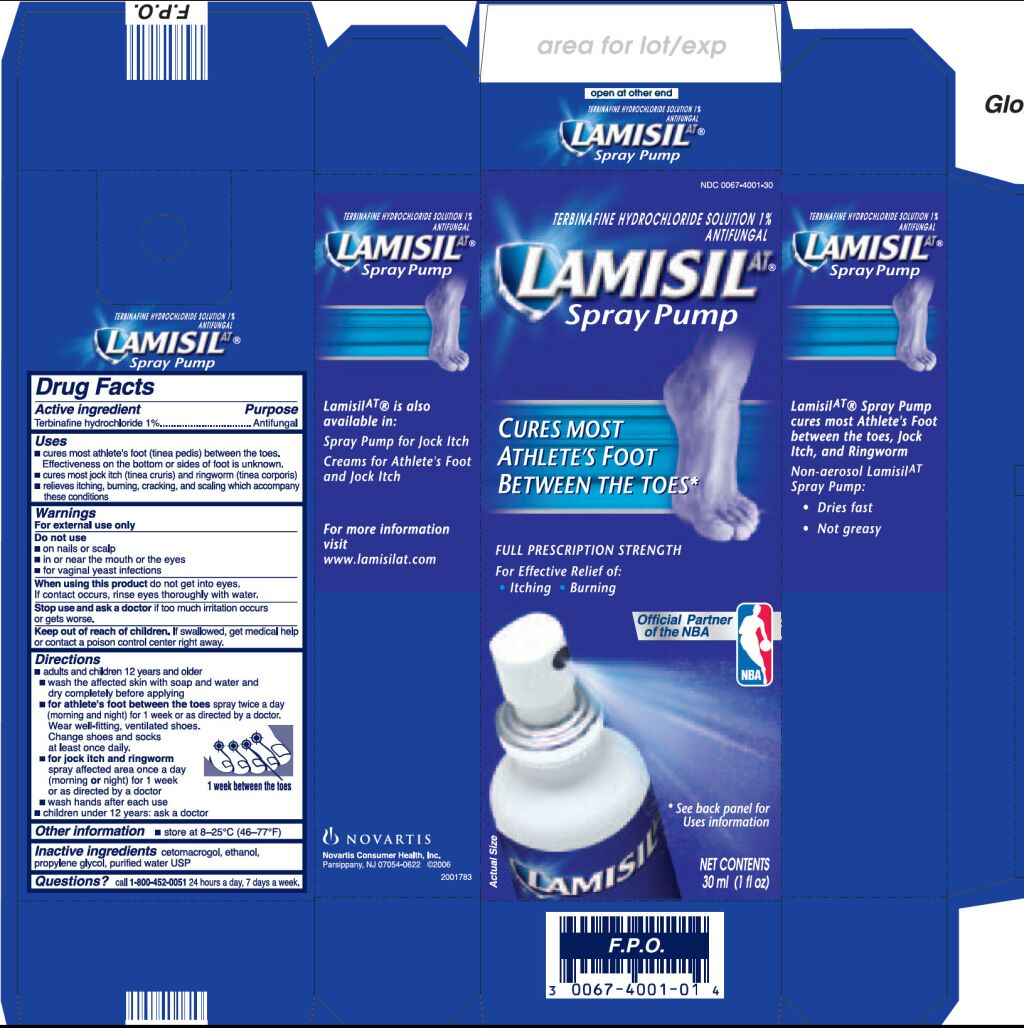
- cures most athlete’s foot (tinea pedis) between the toes. Effectiveness on the bottom or sides of foot is unknown.
- cures most jock itch (tinea cruris) and ringworm (tinea corporis)
- relieves itching, burning, cracking, and scaling which accompany this condition
Warnings
For external use only
Do not use
- on nails or scalp
- in or near the mouth or the eyes
- for vaginal yeast infections
When using this product
do not get into eyes. If contact occurs, rinse eyes thoroughly with water.
Stop use and ask a doctor if
too much irritation occurs or gets worse
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control
Center right away.
Related/similar drugs
clotrimazole topical, ketoconazole topical, terbinafine, miconazole topical, Lamisil
Lamisil AT Spray Dosage and Administration
- adults and children 12 years and over
- wash the affected skin with soap and water and dry completely before applying
-
for athlete’s foot between the toes spray twice a day (morning and night) for 1 week or as directed by a doctor. Wear well-fitting, ventilated shoes. Change shoes and socks at least once daily.
-
for jock itch and ringworm spray affected area once a day (morning or night) for 1 week or as directed by a doctor
- wash hands after each use
- children under 12 years: ask a doctor
Storage and Handling
store at 8° - 25° C (46° - 77° F)
Questions? call 1-800-452-0051
Distributed by: Novartis Consumer Health, Inc.
Parsippany, NJ 07054-0622
Inactive ingredients
cetomacrogol, ethanol, propylene glycol, purified water
LAMISIL AT
terbinafine hydrochloride spray |
|
|
|
|
|
|
|
|
|
|
|
|
More about Lamisil AT Spray (terbinafine topical)
Patient resources
Professional resources
Other formulations
Related treatment guides
Medical Disclaimer